The Johnson & Johnson COVID-19 vaccine was launched on February 27, 2021. It became the third vaccine against coronavirus to get emergency use authorization from FDA (US Food and Drug Administration.) This article will review the Johnson & Johnson vaccine in detail.
Johnson & Johnson Company developed the COVID-19 vaccine working hand in hand with Janssen, its pharmaceutical branch. The project names for this development were Ensemble and Ensemble 2. This vaccine was tested using two different doses with participants being brought on board in September for this last stage. With the testing in clinical trials, a single dose was found to have a good effect. This moved the company to apply for the single-dose with the EUA. The Johnson & Jonson has since become the first COVID-19 vaccine in America to be given as a single dose to adults of 18 years and above.
According to J&J, it was confirmed from the clinical trials that its vaccine is 66% efficient as a single COVID-19 vaccine dose. This vaccine is being used for emergencies not only in the U.S, but also in Bahrain. The Johnson & Johnson pharmaceutical arm, Janssen Pharmaceutica, is based in Belgium and it worked with Beth Israel Deaconess Medical Center in developing this COVID-19 vaccine.
How the Vaccine Works
This is a single-dose vaccine that is adenovirus-based. Adenovirus is a live, yet weakened pathogen and this vaccine is a viral vector type that uses this pathogen and its way of getting the COVID-19 vaccine. A vector is a way of delivering the COVID-19 recombinant vaccine. Such recombinant vaccines require genetic material from the virus, in a small portion, for there to be an immune response.
The COVID-19 genetic material is not replicated in the body. In the vaccination, a particular aspect of the virus can be focused on. It is also safe to use the common recombinant vaccines with large populations. Such vaccines are also safe for people with chronic illnesses and have compromised immunity. They include pneumococcal vaccines and more, with the rabies vaccine for animals being the only commercial adenovirus-based vaccine.
These recombinant vaccines may, however, have disadvantages such as the need for booster shots over time. Another negative issue is that adenovirus-based vaccines may not be very effective when booster doses are administered to a patient. In some cases, we also have people who have virus immunity and this renders the vaccine ineffective. While these are factors to consider, we await to see the effect of the J&J COVID-19 vaccine on vaccinated people.
According to researchers, the coronavirus spike protein gene was added to Adenovirus 26, another virus. Adenoviruses are commonly known to cause colds and flu. The Johnson & Johnson Company used an altered version of adenovirus that can get into cells but not replicate inside the cells or result in illness.
The J&J COVID-19 vaccine is a result of research carried out by the company on adenovirus-vaccines for decades. The first result vaccine by Johnson & Johnson was the Ebola vaccine, which was approved for general use in July. There are still more adenovirus-based vaccines on trial that are being made by the company. In addition to the Johnson & Johnson vaccine, other coronavirus vaccines are also based on adenoviruses. A perfect example is a vaccine made by the University of Oxford and AstraZeneca, which was made using adenovirus from a chimpanzee.
Compared to mRNA vaccines by Pfizer and Moderna, the COVID-19 vaccines that are adenovirus-based are tougher. RNA is more fragile than DNA and the tough protein coat in the adenovirus aids in the protection of the internal genetic material. The Johnson & Johnson vaccine can, therefore, be stored in refrigeration at 2–8°C for a maximum period of three months.
Entering a Cell
The Johnson & Johnson COVID-19 vaccine is injected on the arm, after which adenoviruses build into cells and bolt onto proteins. The cell then submerges the coronavirus and pulls it inside in a bubble. When the virus gets into the bubble, the adenovirus leaves the bubble and moves to the nucleus where the cell’s DNA is stored. Adenovirus moves its DNA into the nucleus and it is created in a way that it cannot duplicate itself. The coronavirus spike protein gene can however be read by the cell and duplicated into the mRNA or messenger RNA molecule.
Spike Proteins
The mRNA molecule moves from the nucleus and the molecules of the cell can read its movement, from where they start forming spike proteins. There are spike proteins produced by the cell that form spikes that move to the cell’s surface and put up their tips. Some of the proteins are also broken into fragments by the vaccinated cells, placing them on their surface. The immune system can thereafter recognize the spike protein remains and spikes. The immune system is also aggravated by the adenovirus by turning the cell’s alarm systems on. To activate the nearby immune cells, the cell sends warning signals and in raising the signals, the Johnson & Johnson COVID-19 vaccine makes the immune system react to the spike proteins more vigorously.
Identifying the Intruder
As soon as vaccinated cells die, there are protein fragments and spike proteins contained in the debris. These can be consumed by an antigen-presenting cell, which is a type of immune cell. On its surface, this cell presents some remains of the spike protein. There are other cells, helper T cells, that identify the spike protein fragments, and they can raise alarm and gather other immune cells in fighting the infection.
Antibodies
B immune cells can get in contact with the coronavirus spikes on the vaccinated cells or get into contact with remains of spike proteins floating freely on the surface. Few B cells might manage to get locked on the spike proteins. If these few B cells get activated by the helper T cells, they begin to multiply and give out antibodies that attack the spike protein.
Tackling the Virus
The antibodies that come from the above process can bolt on coronavirus spikes, marking the virus to be destroyed. The antibodies also block the spike proteins from getting attached to other cells, thus preventing infection.
The cells that present the antigens can also trigger a killer T cell, which is a type of immune cell. This killer T cell is efficient in getting cells that are infected with coronavirus and show some spike protein parts and destroying them. As earlier mentioned, the Johnson & Johnson COVID-19 vaccine is administered as one effective dose compared to other vaccines which require to be given twice. It is however still unclear how long the vaccine offers protection to those who receive it. The number of killer T cells and antibodies might begin to go down months after the Johnson & Johnson vaccine is given. Researchers however say that it is likely for information about coronavirus to be retained in the body for as long as decades to come. This is made possible by the existence of special cells in the immune system known as memory B cells and memory T cells.
How effective is the vaccine?
As per the Johnson & Johnson report, a single dose of its COVID-19 vaccine has a 66% effective prevention rate. In the prevention of severe diseases, the COVID-19 vaccine is 85% effective. This was determined 28 days after vaccination. In the trial, the rate of the vaccine’s efficiency against moderate to serious COVID-19 infection was different based on various regions.
This efficiency was 57% in South Africa, 72% in America, and 66% in Latin America. On this, some COVID-19 variants were included. It is also important to note that the Johnson & Johnson COVID-19 vaccine was found to be 100% effective in protection against COVID-19 hospitalization and death.
When can people begin accessing the vaccine?
The Johnson & Johnson COVID-19 vaccine is already being distributed. This is after it received authorization for emergency use by the FDA. The Johnson & Johnson Company got into an agreement with the federal government to produce 100 million doses of the vaccine at $10 for each dose. The contract included an option of buying 200 million more doses.
The US federal government has also partnered with Merck to help in fastening the production of this COVID-19 vaccine. The Biomedical Advanced Research and Development Authority is set to give Merck $268.8 million to ensure that the vaccine’s manufacturing facilities are available.
Vaccination efforts are being managed by CDC (Centers for Disease Control and Prevention. The agency is also the one officially mandated to handle all COVID-19 vaccine orders and distribution regardless of who the manufacturer is. The organization’s Advisory Committee on Immunization Practices (ACIP) has given recommendations on how the supplying of vaccines will be prioritized. This prioritization can however differ depending on individual states.
According to the recommendations by CDC, healthcare workers will be given priority together with people who live in long-term care facilities. There are also other priority groups. CDC stated that America has more than 1.3 million people living in long-term care facilities and more than 18 million healthcare workers. It will therefore take months to supply the vaccine to all the targeted priority groups and information will continue to be rolled out with time by the CDC. The US government hopes that there will be enough vaccines available for the entire population in America by the end of May.
There is however no detailed information particularly on the exact date when all American residents will get the vaccine and where to get it from. Health departments at the national and state level are working on ensuring that available vaccines are distributed accordingly. As the vaccine becomes more available, American residents can access it from physician offices and other vaccine retailers countrywide.
The Cost of a COVID-19 Vaccine
As soon as the COVID-19 vaccine is fully available, there are doses that the US federal government will purchase. These doses will be freely available for American citizens. However, there may be a vaccine administration fee applied by the facility or agency administering it. Compensation will be offered by insurance policies and Public Health programs for any costs incurred in the vaccination process.
Who is eligible for the Johnson & Johnson vaccine?
Most of the COVID-19 vaccination efforts are focusing on adults. For children and teens, are expected to have suitable vaccines later in the year. It is the same case with the Johnson & Johnson COVID-19 vaccine that is authorized for adult use. The vaccine’s trials were carried out on adults aged 18 years and above.
Johnson & Johnson Vaccine Side Effects
The coronavirus is still a new thing and scientists are continuing to study it keenly. The available vaccines may still miss a lot of facts and information that might be available in the future, following additional research. Participants who took place in the vaccine’s trial were requested to record any reactions they might have encountered after receiving the vaccine. One might experience side effects or adverse reactions. Side effects are physical reactions to a type of medication whereas adverse reactions are caused by the vaccine.
According to the company’s safety information, some of the reactions that vaccinated people experience include pain, swelling, and redness at the injection site, muscle aches, headache, fatigue, fever, and nausea. According to the FDA, people with compromised immunity are also likely to have zero immune response to the vaccine.
Development and Funding of the Vaccine
The U.S. government earlier initiated a public-private partnership referred to as Operation Warp Speed, aimed at fast-tracking the development, manufacturing, and distribution of a covid19 vaccine. As part of this initiative, Johnson & Johnson co-founded the development of the company’s COVID-19 vaccine.
Coronavirus
In this section we review a piece of the coronavirus to help you understand better how the virus works. The SARS-CoV-2 virus uses the proteins that cover it, to enter human cells. The proteins are commonly known as spike proteins and they strategically target possible virus treatments and vaccines. The Johnson & Johnson COVID-19 vaccine focuses on the COVID-19 genetic instructions to build this spike protein. Contrary to the Moderna and Pfizer-BioNTech vaccines where instructions are stored in one RNA strand, this vaccine uses double-stranded DNA.
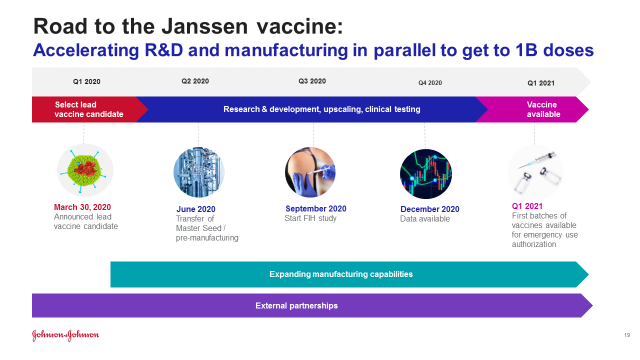
Johnson & Johnson Vaccine Timeline
It was in January 2020 when the Johnson & Johnson Company began to work on the COVID-19 virus vaccine. In March 2020, the US government offered the company $456 million to aid in the development and production of the vaccine. The first and second phase of the vaccine’s trial began in July 2020 and it involved one and not two doses.
The US federal government later agreed to pay $1 billion for 100 million doses of the vaccine once it is approved for use. The third phase of the vaccine’s trial was launched in September 2020. It was in October 2020 when the European Union received a deal to acquire 200 million doses when the vaccine is approved. During the phase three trial, there occurred an adverse reaction on one of the volunteers in the trial. This led to the third phase trial being paused on 12 October and it resumed on 23 October.
There was a second phase three trial that was launched on 16 November to test two doses of the vaccine instead of one and see the effects. Nearly 45, 000 people enrolled for the vaccine’s phase three trial with the trial results being expected in January 2021. The company has the goal of producing a minimum of a billion doses in 2021.
As per the results of the trials released on February 24, 2021, the Johnson & Johnson COVID-19 vaccine was found to be 72% efficient in the United States. This followed authorization of the vaccine by the FDA for emergency use on February 27, 2021, becoming the third coronavirus vaccine to be produced in America. This vaccine has so far received emergency authorization from the US Food and Drug Administration. It has been recommended by the Centers for Disease Control and Prevention with the US federal government laying down plans to begin its immediate distribution.
Differences Between The Three Approved COVID-19 vaccines
Currently, two vaccines are being distributed in the United States. One has been produced by Moderna while the second one has been produced by Pfizer in conjunction with its German-based partner BioNTech. These first two vaccines will be different from the new Johnson & Johnson COVID-19 vaccine in various ways.
Number of Vaccine Doses
The latest Johnson & Johnson COVID-19 vaccine is made to be administered as one complete dose. When one receives this dose, therefore, the person does not require check-up visits and there is no need to return for a second shot or dose. This means that you will only receive it once and it is proven to be efficient.
For the Moderna and Pfizer COVID-19 vaccines, however, they are made to be given in two doses. You should receive the second Pfizer vaccine dose after three weeks of receiving the first dose and four weeks for the Moderna vaccine. Discussions have been there on the possibility of giving these two vaccines as one dose other than two separate ones. The other proposal is to have the time between doses extended to allow more people to receive the first one for some little protection from the virus.
According to the FDA authorization, however, these vaccines must be administered in two doses and this has been supported by others including experts. According to the chief medical adviser at White House, Dr. Fauci, a single dose of the Moderna or Pfizer vaccine might only offer partial and not complete protection.
The Johnson & Johnson vaccine was however tested and it proved to be able to offer proper protection one-time with a single dose. There are studies however to see if getting two Johnson & Johnson vaccines would mean more protection from the virus.
Efficacy Difference
The Johnson & Johnson vaccine is very efficient. The Moderna and Pfizer vaccines are also tested and determined to be efficient. From clinical trials, the vaccines showed high efficiency of 95% with the Pfizer vaccine in Israel indicating the same efficacy rate.
The Johnson & Johnson vaccine was tested in various populations. There were tests in Latin America, the US, and South Africa. These tests were carried out later during the pandemic and when the Modern and Pfizer vaccines were already in use. The Pfizer vaccine was tried on 43000 people from various countries including South Africa, Brazil, Turkey, Argentina, and Germany. For the Moderna vaccine testing was carried on 30,000 people from the United States.
According to experts, all of the three approved vaccines are efficient in their way and this is important. The differences in efficiency may result in many speculations about the vaccine, with many having a preference.
Quicker Protection
When one receives the Johnson & Johnson COVID-19 vaccine, the body begins to get protection against moderate and severe diseases approximately two weeks after vaccination. There was a great decline in the number of COVID-19 deaths by about four weeks of the Johnson & Johnson vaccine. Study reports are also showing that the very first Moderna and Pfizer vaccines offer a great deal of protection to those who receive them. These people can however only receive complete protection from the virus nearly two weeks after they receive the second dose, making it approximately 5 weeks after the first.
Difference In Technology
There is a brand-new technology that the Pfizer and Moderna vaccines use; it is known as mRNA or messenger RNA. Using this, the vaccines deliver genetic content into cells through fatty elements directly. Cells then take up the genetic code in the arm muscle and genetic instructions are issued to create tiny protein pieces that resemble the coronavirus. The protein pieces trigger a response in the immune system that leads to immune cells and antibodies that master how the virus looks like. The antibodies and immune cells will be ready to respond to any fresh infection of a similar kind. This means that the Pfizer and Moderna COVID-19 vaccines can offer protection against any coronavirus variant and this is quite effective.
On the other hand, the J&J vaccine operates using viral vector technology. In this vaccine, adenovirus 26 which is a cold virus is made genetically to make it infect cells. The cold vaccine however does not replicate in the cells and also won’t spread in the body leading to cold or flu. Similar to the Moderna and Pfizer vaccines, the Johnson & Johnson vaccine gives genetic instructions in fighting the virus. With the Johnson & Johnson COVID-19 vaccine, the genetic instructions are put in the arm cells by the already weakened coronavirus. This leaves the pieces looking like a section of the coronavirus thus aiding in the fight against the virus.
Vaccine Handling
The Moderna and Pfizer vaccines are carried in little balls of fat that are quite sensitive. The requirements of storing and shipping Pfizer’s vaccine are -112ºF to -76ºF which is equivalent to -80ºC to -60ºC. This was quite tough for many states to meet in the beginning as they needed to get special freezers and enough dry ice. Currently, however, there has been easing of the storage and transportation requirements. The Pfizer vaccine should however still be stored in a refrigerator for a maximum of five days whereby it is used no more than six hours after it is melted and diluted. Important to note is that the Pfizer vaccine must be diluted before use and it must not be shaken. This vaccine should be inverted 10 exact and accurate times to mix it before use. For the Moderna vaccine, storage is under -20ºC refrigeration and it should be handled carefully. The Johnson & Johnson vaccine can however be stored in moderate and simple refrigeration temperatures for as long as three months. This makes the vaccine the easiest to transport and store.
Similarities of the Vaccine
The main similarity among the three COVID-19 vaccines is the fact that none is made using additives. Additives may result in strong reactions for vaccinated people and their absence in the vaccines means that there is minimal risk of an allergic reaction. As per the CDC, there have so far been few cases of anaphylaxis reported and this is the biggest form of a life-threatening allergic reaction. All the reported cases were from people vaccinated with Moderna or Pfizer vaccines and they all got treated fast. From the Johnson & Johnson vaccine, there has only been one such case reported.
All three vaccines focus on the receptor-binding domain in the spike protein. This is where the coronavirus handles cells and should mutation take place in this area, the Johnson & Johnson vaccines and the others too could become less efficient. An advantage is that the three authorized vaccines boost immune response and offer protection even as research continues to be carried out on the coronavirus.